
Gage Lab
Dept. of Molecular and Cell Biology
University of Connecticut - Storrs
Research-Sinorhizobium
Signal transduction via the PTS in S.meliloti
Free-living S. meliloti can utilize a wide variety carbon sources including sugars, amino acids and TCA cycle intermediates. A large body of evidence shows that C4 TCA cycle intermediates are used to fuel and provide reducing equivalents for symbiotic forms of S. meliloti. In addition, these compounds are favored carbon and energy sources for free-living S. meliloti, and are used in preference to many other carbon sources. The phenomenon of utilizing succinate, and other C4-dicarboxylic acids, in preference to other compounds in S. meliloti, is called succinate-mediated catabolite repression (SMCR). Control of SMCR is deeply integrated into the physiology of S. meliloti and the the genes that regulate SMCR also regulate carbon storage, growth and survival, exopolysaccharide synthesis, symbiosis and nitrogen fixation. SMCR is regulated by both the phosphotransferase system (PTS) and by a two-component system. Two PTS proteins are particularly important: Hpr and EIIANtr. Hpr is the central regulator through which all phosphorylation signals travel, and it integrates information about S. meliloti’s physiological state via PTS proteins EINtr and HprK. EIIANtr appears to be the main PTS output signal regulating SMCR and symbiotic functions. Recent work shows that regulation of SMCR is connected to the synthesis of polyhydroxybutyrate (PHB) and that PHB has global effects on gene expression and physiology of S. meliloti.
Signaling by a FATGY two-component sytstem in S. meliloti
Signaling in prokaryotes consists of a chain of events that begins with the detection of signal and ends with a physiological response that is usually adaptive. Two component signaling systems (TCSs) are one of the most common signal transduction systems in prokaryotes. The typical TCS consists of a histidine kinase (HK) and a cognate response regulator (RR), which work to modulate a physiological response. Recently a new family of HKs associated with two component systems was described. These HKs, called HWE kinases, are notable because their kinase domains are different from previously known domains and often signal in response to light, energy, redox or small molecules. HWE kinases work with FATGY RRs that are similar to each other at the sequence level and together define a RR subgroup. One goal of our lab is to better understand physiological and structural aspects of HWE_HK/RR signaling using a model set of proteins, Sma0113(HWE_HK) and Sma0114(RR). These two proteins are encoded in the genome of S. meliloti, and together regulate catabolite repression and carbon storage (PHB synthesis). We have recently published the NMR structure of the RR Sma0114 and found that it is lacking the a4 helix, and instead has a large flexible loop--a feature likely common in FATGY RR structures.
Selected papers - PTS
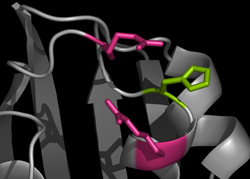
Model of S. meliloti Her showing the phosphorylated Histidine and nearby Arginine residues
HPrK regulates succinate-mediated catabolite repression in the Gram negative symbiont Sinorhizobium meliloti. J. Bacteriol. 191:298-309 get pdf
Sinorhizobium meliloti mutants lacking phosphotransferase system enzyme HPr or EIIA are altered in diverse processes, including carbon metabolism, cobalt requirements, and succinoglycan production. J. Bacteriol. 190:2947 get pdf
a-galactoside uptake in Rhizobium meliloti: isolation and characterization of agpA, a gene encoding a periplasmic binding protein required for melibiose and raffinose utilization. J. Bacteriol. 180: 5739 - 5748 get pdf
Selected papers - Sma0113/0114

Nuclear magnetic resonance structure and dynamics of the response regulator Sma0114 from Sinorhizobium meliloti. Biochemistry 51: 6932. get pdf
Characterization of a two-component regulatory system which regulates succinate-mediated catabolite repression in Sinorhizobium meliloti. J Bacteriol. 192: 5725 get pdf